Enhancing pharmaceutical process design with simulation

How digitalization is transforming pharmaceutical scale-up from lab to production
The pharmaceutical industry has always been a challenging business due to its long incubation and high R& D costs. Today, there is even more pressure as newer drug types are being investigated. The primary aim of pharmaceuticals is to provide medical treatment for chronically or acutely sick patients. The industry is still striving for breakthroughs in diseases which claim lives too early – this means the development of more innovative drugs that add another layer of complexity.
Technological advances such as computer modeling, AI and initiatives such as Quality by Design (QbD) are enabling the discovery of new therapies and delivery platforms, but these often require new and more intricate manufacturing techniques which make it difficult to bring them to the market quickly and cost-effectively. And as if this wasn’t enough, pharmaceutical companies must deal with scale-up challenges and the pressure to make businesses fully sustainable as well as profitable. With all these challenges, the industry needs to adopt new approaches to continue to push the boundaries of medicine while keeping costs down and getting new treatments to patients as quickly as possible.
Handling length-scale, time-scale, and multiphysics complexity
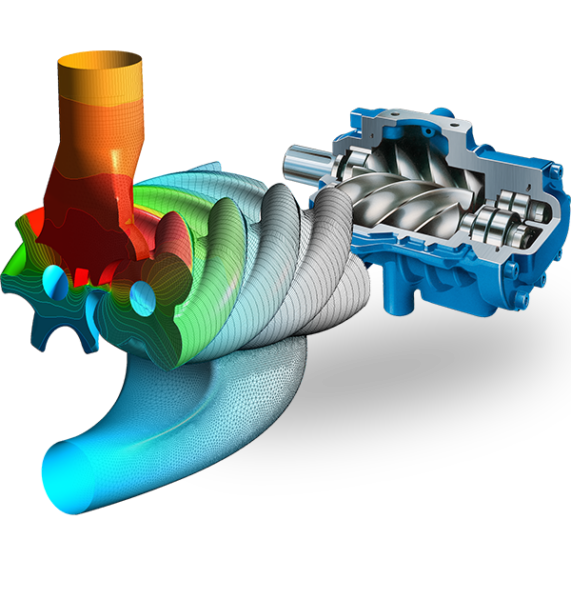
Simulation is a vital tool for the pharmaceutical industry due to the unique and detailed information it can provide for many operations with fluids, particles and solid mechanics, often beyond what is either experimentally possible using sensors and traditional empirical guides. However, an extra complication of simulation in the pharmaceutical industry is the different length-scales, time-scales, and multiphysics involved in the drug manufacturing process. Multiple tools are needed to simulate these which, if used separately, leads to the creation of data silos.
Each new pharmaceutical product developed requires increasingly complex and costly physical experiments. These experiments generate large volumes of fragmented data that must be contextualized and analyzed to produce results. No easy task when teams are working in separate silos without any easy methods for knowledge sharing and collaboration to make regulatory submission and manufacturing simpler.
Digitalization is key
Hence the question arises – ‘how can you rapidly design a robust manufacturing process that enables more efficient scale-up from lab to production?’

The key is digitalization of the entire product lifecycle, from drug discovery to commercial manufacturing, enabling a continuous optimization loop that feeds data and insights from clinical trials and manufacturing back into future research and development. By leveraging simulation solutions, pharmaceutical companies are better placed to accelerate recipe development, enhance collaboration and drug manufacturability, reducing time-to-market and saving on resource costs.
Bridging the gaps to address pharmaceutical complexity
Building a digital twin of products and processes allows companies to combine real-world data with simulated data to design predictive and prescriptive models. Designing and scaling up recipes from laboratory to clinical trials and commercial manufacturing with an ISA-88 guided Enterprise Recipe Management (ERM) approach enables knowledge-driven digital recipe transformation.
In this way, manufacturers can increase the robustness, cost-efficiency, and sustainability of production by reducing the use of raw materials and optimizing equipment usage.
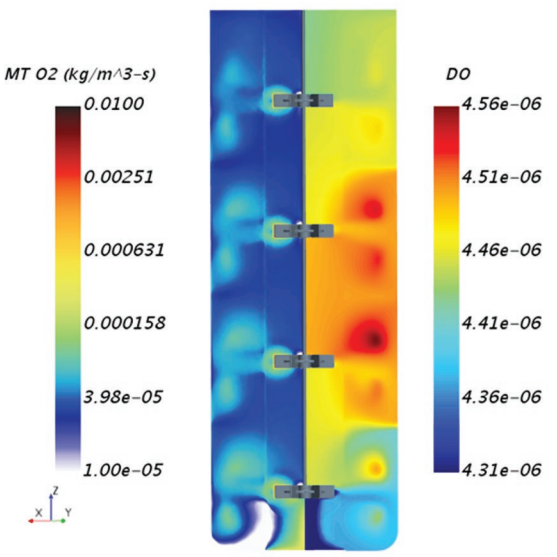
A CFD simulation of a gas-liquid bioreactor modeled using Siemens Simcenter STAR-CCM+
However, simulation alone is not the answer to meeting the modern challenges faced by the pharmaceutical industry. Pharma manufacturers need to be able to derive insights from simulation quickly and capture this data in an efficient way to make informed decisions in real-time. This is why products within the Simcenter portfolio are built to combine multiscale and multiphysics simulation. This helps bridge the gaps between the different development stages that make up the complete pharmaceutical product lifecycle.
The value of the Executable Digital Twin (xDT)
An xDT is a reduced physics-based model that is simple enough to be run in near real-time, while still accurately representing the process using state-of-the-art mathematical techniques with these models to analyze and optimize process design or operation. The value of this optimization is assured for the lifetime of production, amounting to billions of dollars in some cases.
GSK, a global biopharmaceutical company utilized Simcenter solutions to develop the first virtual replica of a vaccine process which reduced the development time of vaccines by 25%. The Executable Digital Twin (xDT) enabled them to virtually test production processes at every stage of development, collecting real-time data with virtual sensors that delivered vital insights. As well as getting the vaccines to market sooner, this saved significant numbers of batches that would otherwise have been wasted. Similar techniques have since been used by a German pharmaceutical company, BioNTech SE in the production of crucial COVID-19 vaccines to help keep the pandemic under control.
More than simulation
Modeling the complexity of modern pharmaceutical development and production is essential to understanding the geometry, physics, and anything else at play that may influence performance. Simulation then enables the exploration of all the possibilities – virtual models allow engineers to experiment with more freedom and without the constraints imposed by physical testing.
While time-to-market and global market presence are absolute priorities for pharmaceutical companies, it is crucial not to neglect process optimization. Simulation insights will benefit both process optimization and faster design space exploration in an increasingly competitive market.
Simcenter Simulation and Test solutions help pharmaceutical companies leverage the opportunities of digitalization and digital twin technologies
Just as important is the seamless integration of different Simcenter tools that allows development in different functional areas to continue simultaneously. Close collaboration between teams ensures all are aware of the latest changes so that decisions are optimized across the entire process, not for individual components. Combined with the latest process capture tools, workflow automation, and options for running the software on high performance cloud platforms, Simcenter makes development much faster and more accurate.
To find out more about how Simcenter is transforming the pharmaceutical industry, read our White Paper here: