Efficient laundry washing at 30°C – Designing low-temperature detergents with Computational Chemistry

I consider myself a good cook. I come from a family where preparing meals together has always been an integral part of our life. I learned how to use the respective kitchen tools and I thoroughly learned how to chop, mix, taste, in short: cook. But there is one thing. Just a tiny little thing that for some reason I haven’t mastered.
I tried everything, really!
And yet, I have pictures of me, at all ages eating or baking chocolate cakes, and sure enough, there is chocolate on my forehead and all over my clothes for some reason! Meanwhile, I think it’s genetic. And today, I’Il frankly admit it: I cannot bake without ending with chocolate everywhere on me.


Suffice to say that I have an entire collection of aprons in my kitchen which do a regular trip to the washing machine, to say the least. And while I have given up on getting rid of the chocolate on my aprons in the first place (and I assume some baking peers or parents know what I mean), this surrender in return implies a huge challenge on a whole industry:
Cold days, hot challenge
Not so long ago, clothes were washed at 40 ⁰C. Today, we all routinely wash them at 30 ⁰C. We do this both to protect the fiber, but also and mainly for ecological reasons. This small decrease in temperature sounds trivial to us users, but it had a great impact on the detergent industry. Changing the operating conditions by just those 10 degrees can drastically degrade the efficiency of a given detergent formulation. And the core question to all these companies is, are their detergents still as efficient at 30 ⁰C than at 40⁰C?
To find out, follow me into the world of atoms and molecules, into the world of computational chemistry. Here lies the magic of how those aprons go in the machine dirty and then come back all clean, ready for the next chocolate adventure!
And while we are at it, let’s see if computational chemistry can help the detergent designing companies and ultimately you to get rid of that chocolate – even at 30 degrees Celsius? It is a hot challenge, served cold.
Origins of a soap box opera
But let’s start with the origins of the soap box opera. At the beginning there was water. And soon there was soap. Soap, essentially a salt of fatty acid, or a type of oil, that has gone through saponification, and maybe some dye and fragrances. Soap molecules both like oil and water, making them perfect for cleaning our hands when coming home after a long day.
But for chocolate on your apron? Ask your grandparents. You better not cry over spilled chocolate. You needed to step up your soap game.

Laundry detergents – and cleaning clothes became (almost) a breeze
It’s 1907. Henkel introduces laundry detergents with their famous Persil brand. This revolutionized how we washed clothes as it was not a simple soap anymore, like the ones we have been using for 5000 years. It was a mix of different molecules that brought together were more powerful than a simple piece of soap! Other companies followed quickly like Proctor and Gamble (P&G) who added surfactants, a type of molecule that both likes oil and water, to the mix.
Today, laundry detergents are a mix of builders, or water softeners, surfactants, sometimes bleach, enzymes that take care of the difficult stains, fragrances, dyes, etc.
Quite a complex formulation compared to the good old soap. A powerful formulation. And it worked. The modern detergent conquered the world together with the washing machines. And out of a sudden cleaning clothes became (almost) a breeze.
How to clean clothes at ever-decreasing water temperatures
But if you think what follows was a piece of cake, you shall be corrected: 90 degrees Celsius, 60 degrees Celsius, then 40 degrees Celsius and ultimately 30 degrees Celsius. Over and over, the desire to lower energy consumption and hence the electricity bill and -today more than ever – the CO2 footprint, forced chemical companies to refine their recipe of low-temperature detergents to clean clothes at ever-decreasing water temperatures.

This, I can tell you as a computational chemistry engineer, is a task far from trivial (and another league compared to my own chocolate-free-apron challenge).
So today more than ever, companies need an answer on how could they modify their formulation quickly? Indeed, keeping pace with the competition is no small feat. The good news is that today they have Computational Chemistry as a powerful virtual screening tool. Because computational chemistry looks at materials at the atomistic level, it really can give you a new perspective on things. This happened when I was asked to work on laundry detergents together with one of our customers.
And so the challenge was clear: there was a list of detergent formulations and an experimental setup, and we together with our client had to find a way to model all of that; to then asses the performance under lowering temperature and ultimately find ways to have a detergent work reliably at 30 degrees Celsius.
The devil in the (washing) details
But here is the catch, there was no way we could solve this problem straight away. We had to be creative.
To understand the challenge, it‘s time to dig into the techniques here. Computational chemistry is in reality a collection of techniques that allow us to study the micro-world at different levels depending on what we want to learn. For laundry detergent, we are typically looking at the mesoscale where molecules are not represented with their atoms anymore, but by beads which each represent a few atoms themselves. By doing that, we can go from simulating the behavior of a thousand molecules to tens of thousands, and we can model extremely complex systems like the slurry drying of a cathode for battery electrode design, or the performance of a complex washing detergent formulation.
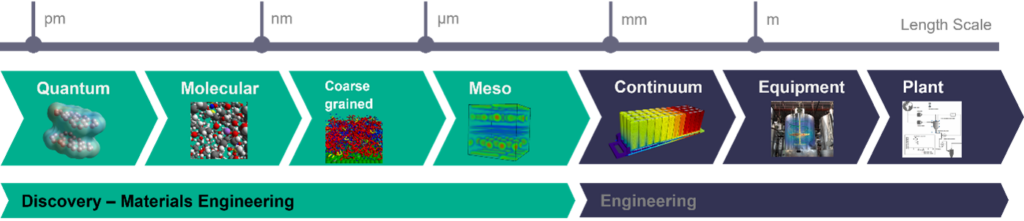
Our method of choice is called Dissipative Particle Dynamics (DPD). It’s been around 30 years and is particularly adapted to complex fluid simulation with typically polymers or surfactants.
But what interests us in the project at hand is – luckily – a little simpler, we want to clean a stain with the formulation of a few molecules that we have. Looks simple enough, we thought, but there was something that we did not foresee: the stain is modeled by a solid crystal and that is not something that we can just routinely model with our classic DPD methodology. DPD can only model the liquid phase. We had to think of something.
The Cubic Potential – one to rule them all
If we look at the most common DPD potential, it is purely repulsive, molecules in liquid state can be nicely described with a repulsive potential and so can gaseous states. But solids, no way. The nature of a solid is characterized by a cohesive force between the molecules that form the chrystal or amorphous structure (that’s the whole point why it is a solid). And – unfortunately- that’s why we cannot model multiphase systems such as a stain on a T-shirt that we want to clean with the existing “repulsive only” potential.
So why not just include an attractivity in the potential like in the Lennard-Jones potential?
That’s just what we did, and that’s how the Cubic Potential was born.
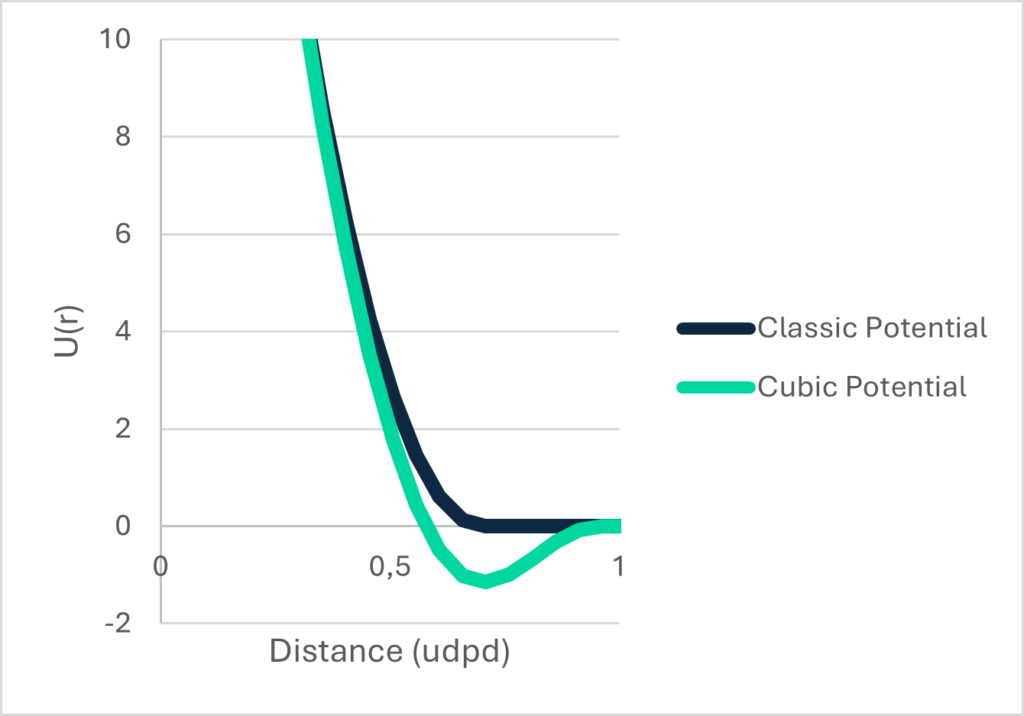
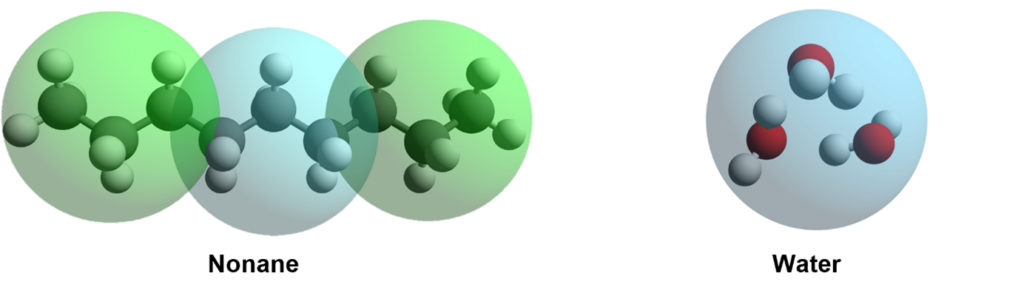
Well, “Just” is a bit misleading. It’s actually more difficult than you would guess. We first started to validate it against water.

If we could reproduce the most elemental properties of water, we could develop it further. The first targeted property was the ability to model the phase transition of water with temperature.
The addition of the attractive well allows the water molecule to aggregate and crystallize if the temperature decreases, but also to transform into a gas form as the temperature reaches water boiling point of 100 ⁰C.
As we successfully reproduced the phase transition of water with temperature, we extended our test set to alkanes. As the graphs below show we were also successful in predicting the phase change and density as a function of the molecular weight.
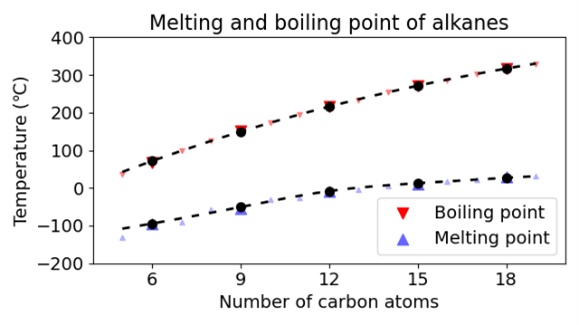
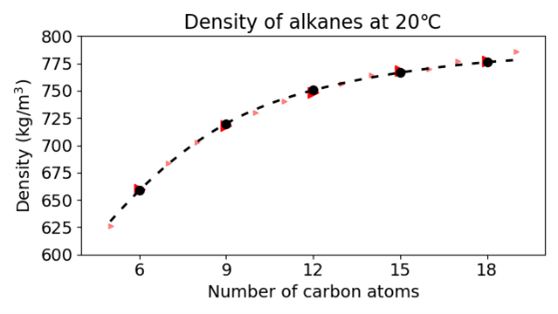
Washing Laundry with the Cubic Potential
Strong with those validation sets, we put the Cubic potential to the test and used it on our detergent solutions. We created a DPD simulation where we put some models for a stain, the detergent solution, and just “washed”.
After a first test we altered the detergent formulation and washed again. As you can see, we were successful in modeling a detergent that washes, but also a detergent that didn’t:
We were able to distinguish a performing low-temperature detergent from on that does not. This was a huge success for our customer who is now equipped to design the next-generation of low-temperature active, more sustainable detergents.
Simulating multiphase systems is now a breeze
But it’s not just about washing clothes. Across any industry and applications: If you are facing a multiphase challenge, where the prediction of phase transitions on a molecular level is of fundamental importance to design a better active material – the new method can mean an upcoming success for you: With the release of Simcenter Culgi 2405 we integrated the Cubic potential in the automated fragmentation and parameterization workflow. This is expanding your ability to simulate any multiphase system, capturing systems of coexisting fluids and solids; at any temperature, and with one parameter set. As such the new potential also enables the correct prediction of all relevant phase transitions solid/liquid, liquid/gas, solid/gas within a single simulation. It removes the dependency of the parameterization on the temperature: While classic DPD required one parameterization set per temperature the Cubic potential is offering one parameterization across all temperatures, significantly reducing the parametrization effort.
For me it means, I will keep on baking my chocolate cakes; with no mercy for the aprons.
PS: Do not try to cook or bake in the washing machine, it’s a matter of chemistry!